Sir William Lawrence Bragg, was an Australian-born British physicist and X-ray crystallographer, discoverer (1912) of Bragg's law of X-ray diffraction, which is basic for the determination of crystal structure. He was joint recipient of the Nobel Prize in Physics in 1915, "For their services in the analysis of crystal structure by means of X-rays"; an important step in the development of X-ray crystallography.
Lawrence Bragg in 1915
Portrait of William Lawrence Bragg taken when he was around 40 years old.
Bragg Family Blue Plaque Leeds
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information.
A powder X-ray diffractometer in motion
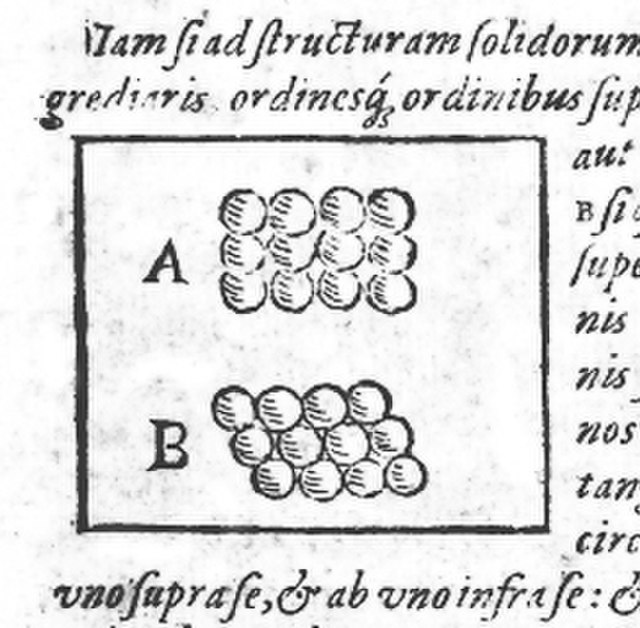
Drawing of square (A) and hexagonal (B) packing from Kepler's work, Strena seu de Nive Sexangula.
One of the copper sulfate X-ray interference patterns published in Von Laue's 1912 paper.
Although diamonds (top left) and graphite (top right) are identical in chemical composition—being both pure carbon—X-ray crystallography revealed the arrangement of their atoms (bottom). In diamond, the carbon atoms are arranged tetrahedrally and held together by single covalent bonds. By contrast, graphite is composed of stacked sheets. Within the sheet, the bonding is covalent and has hexagonal symmetry, but there are no covalent bonds between the sheets.