Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH.
The remains of the 16th century carrack Mary Rose undergoing conservation treatment with PEG in the 1980s
Terra cotta warrior, showing traces of original color
Polyethylene oxide (PEO, Mw 4 kDa) nanometric crystallites (4 nm)
Polyethylene glycol 400, pharmaceutical quality
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information.
A powder X-ray diffractometer in motion
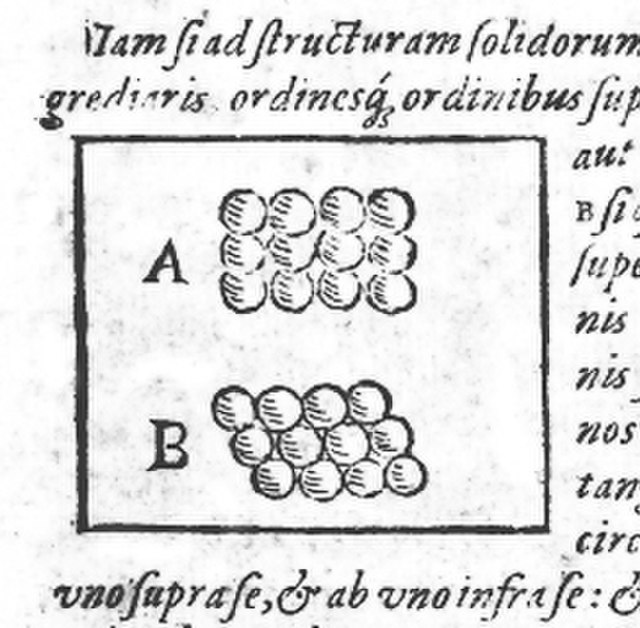
Drawing of square (A) and hexagonal (B) packing from Kepler's work, Strena seu de Nive Sexangula.
One of the copper sulfate X-ray interference patterns published in Von Laue's 1912 paper.
Although diamonds (top left) and graphite (top right) are identical in chemical composition—being both pure carbon—X-ray crystallography revealed the arrangement of their atoms (bottom). In diamond, the carbon atoms are arranged tetrahedrally and held together by single covalent bonds. By contrast, graphite is composed of stacked sheets. Within the sheet, the bonding is covalent and has hexagonal symmetry, but there are no covalent bonds between the sheets.