Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C16H10. This yellow-green solid is the smallest peri-fused PAH. Pyrene forms during incomplete combustion of organic compounds.
Pyrene
STM image of self-assembled Br4Py molecules on Au(111) surface (top) and its model (bottom; pink spheres are Br atoms).
Image: Br 4Py self assembly on Au
Polycyclic aromatic hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.
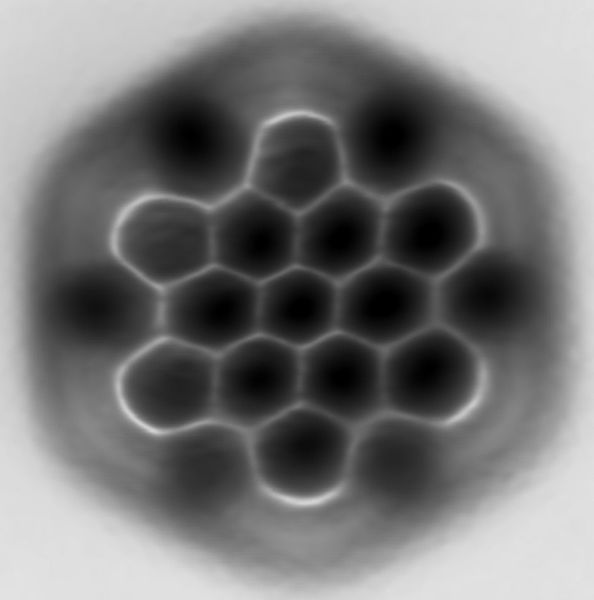
Three representations of hexabenzocoronene, a polycyclic aromatic hydrocarbon. Top: standard line-angle schematic, where carbon atoms are represented by the vertices of the hexagons and hydrogen atoms are inferred. Middle: ball-and-stick model showing all carbon and hydrogen atoms. Bottom: atomic force microscopy image.
A wood-burning open-air cooking stove. Smoke from solid fuels like wood is a large source of PAHs globally.
Smog in Cairo. Particulate air pollution, including smog, is a substantial cause of human exposure to PAHs.
Crude oil on a beach after a 2007 oil spill in Korea.