Rhodopsin-like receptors are a family of proteins that comprise the largest group of G protein-coupled receptors.
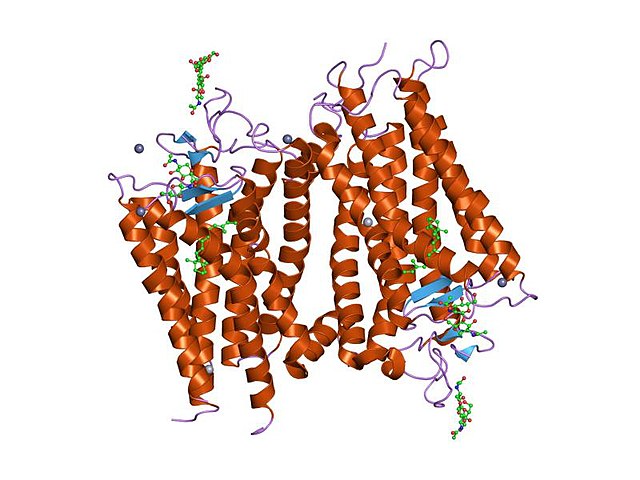
Structure of rhodopsin: A G protein-coupled receptor.
G protein-coupled receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

Cartoon depicting the basic concept of GPCR conformational activation. Ligand binding disrupts an ionic lock between the E/DRY motif of TM-3 and acidic residues of TM-6. As a result, the GPCR reorganizes to allow activation of G-alpha proteins. The "side perspective" is a view from above and to the side of the GPCR as it is set in the plasma membrane (the membrane lipids have been omitted for clarity). The incorrectly labelled "intracellular perspective" shows an extracellular view looking down at the plasma membrane from outside the cell.
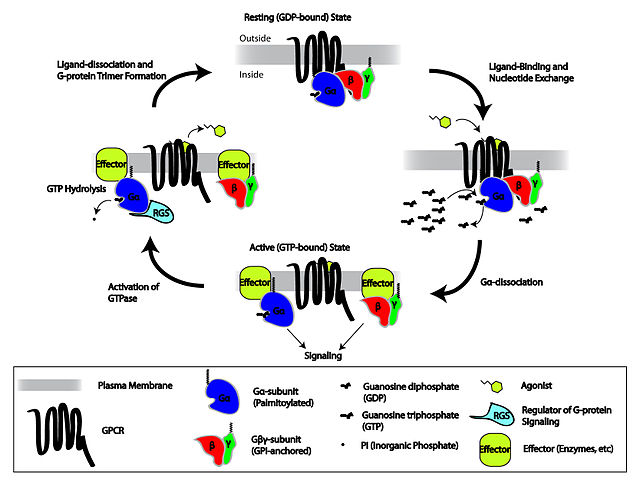
Cartoon depicting the heterotrimeric G-protein activation/deactivation cycle in the context of GPCR signaling
The effect of Rs and Gs in cAMP signal pathway
The effect of Ri and Gi in cAMP signal pathway