Smouldering or smoldering is the slow, flameless form of combustion, sustained by the heat evolved when oxygen directly attacks the surface of a condensed-phase fuel. Many solid materials can sustain a smouldering reaction, including coal, cellulose, wood, cotton, tobacco, cannabis, peat, plant litter, humus, synthetic foams, charring polymers including polyurethane foam and some types of dust. Common examples of smouldering phenomena are the initiation of residential fires on upholstered furniture by weak heat sources, and the persistent combustion of biomass behind the flaming front of wildfires.
Smouldering combustion in glowing embers of barbecue coal briquettes
A smouldering cigarette.
Polyurethane foam sample from the NASA smouldering experiments.
Smoke and pollution from fires in Borneo, 1997.
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science.
The flames caused as a result of a fuel undergoing combustion (burning)
Air pollution abatement equipment provides combustion control for industrial processes.
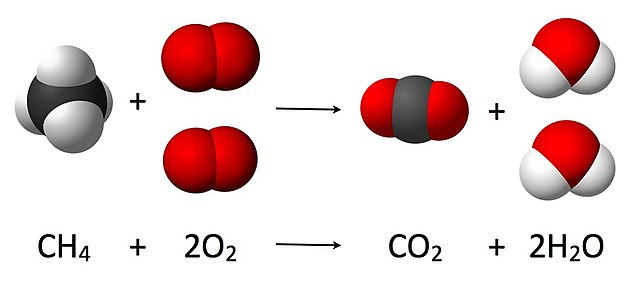
The combustion of methane, a hydrocarbon
Colourized gray-scale composite image of the individual frames from a video of a backlit fuel droplet burning in microgravity