Titanium dioxide, also known as titanium(IV) oxide or titania, is the inorganic compound with the chemical formula TiO2. When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble in water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion.
Titanium dioxide
Synthetic single crystals of TiO2, ca. 2–3 mm in size, cut from a larger plate
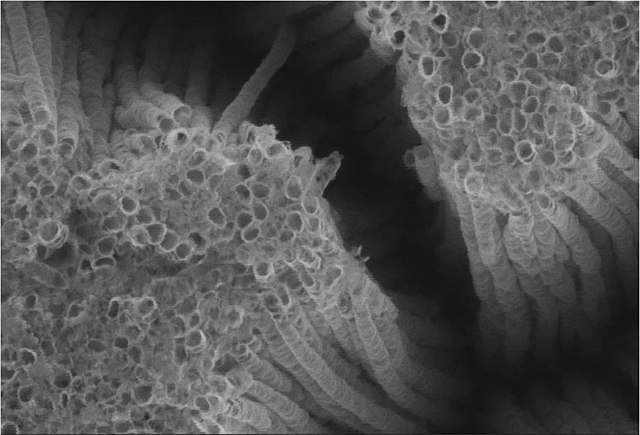
Titanium oxide nanotubes, SEM image
Nanotubes of titanium dioxide (TiO2-Nt) obtained by electrochemical synthesis. The SEM image shows an array of vertical self-ordered TiO2-Nt with closed bottom ends of tubes.
A pigment is a powder used to add color or change visual appearance. Pigments are completely or nearly insoluble and chemically unreactive in water or another medium; in contrast, dyes are colored substances which are soluble or go into solution at some stage in their use. Dyes are often organic compounds whereas pigments are often inorganic. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli.
Pigments for sale at a market stall in Goa, India
The Milkmaid by Johannes Vermeer (c. 1658). Vermeer was lavish in his choice of expensive pigments, including lead-tin yellow, natural ultramarine, and madder lake, as shown in the vibrant painting.
Titian used the historic pigment vermilion to create the reds in the oil painting of Assunta, completed c. 1518.
Miracle of the Slave by Tintoretto (c. 1548). The son of a master dyer, Tintoretto used Carmine Red Lake pigment, derived from the cochineal insect, to achieve dramatic color effects.