Titin is a protein that in humans is encoded by the TTN gene. Titin is a protein, over 1 µm in length, that functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences. These domains unfold when the protein is stretched and refold when the tension is removed.
Titin Ig domains. a) Schematic of part of a sarcomere b) Structure of Ig domains c) Topology of Ig domains.
Reconstruction of the thin (green) and thick filament from mammalian cardiac tissue. Myosin is in blue, MyBP-C is in yellow, and titin is in two shades of red (dark red for titin-alpha and light red for titin-beta).
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.
John Kendrew with model of myoglobin in progress
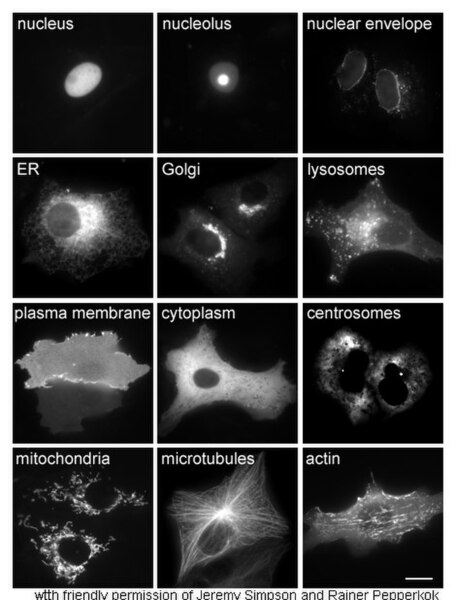
Proteins in different cellular compartments and structures tagged with green fluorescent protein (here, white)
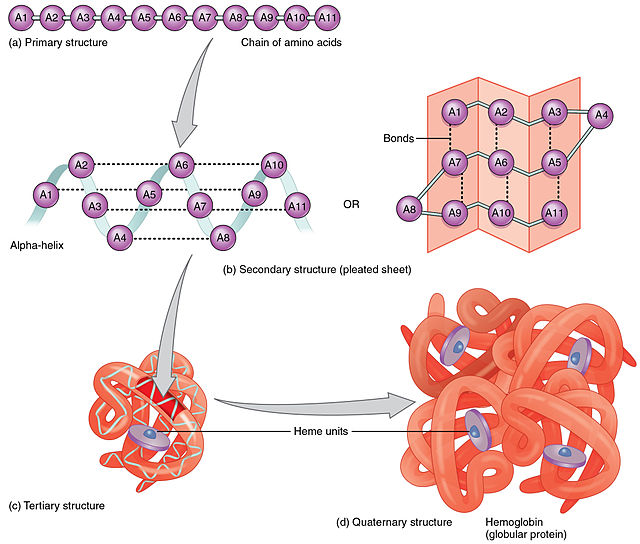
Constituent amino-acids can be analyzed to predict secondary, tertiary and quaternary protein structure, in this case hemoglobin containing heme units